The following paragraphs are an excerpt from the breakthrough paper “Anaerobic ammonium oxidation discovered in a denitrifying fluidized bed reactor”.
Before Anammox - the problem:
Nitrogen removal from wastewater is critical for safeguarding sensitive water bodies. Nitrogen is often a rate-limiting compound for algae growth in surface water bodies. Nitrogen in wastewater when discharged into a water body, it makes it eutrophic. Eutrophication further leads to algae blooms.
Conventional biological nitrogen removal is a two-step process as discussed in FAQ-8. The first step is nitrification and the second step is the denitrification process. Nitrification in itself is often observed as a two-step process. The first step involves the conversion of ammonium into nitrite by ammonia-oxidizing bacteria/archaea. While the second step involves the conversion of nitrite to nitrate by nitrite-oxidizing bacteria. The first step of nitrification is a bottleneck, as the ammonium oxidizers are slow-growing autotrophs. And they compete with fast-growing heterotrophs for oxygen (electron acceptor). The denitrification process involves the conversion of produced nitrate to nitrogen gas. The bottleneck with the denitrification process is that it requires a carbon source (electron donor) and must be supplied. Nitrification and denitrification require different redox conditions i.e. aerobic and anoxic respectively. In wastewater treatment plants, separate chambers are required for each of these processes and the wastewater is cyclically pumped through these chambers.
Summary of the problems with conventional nitrification-denitrification:
1. High requirement of oxygen for nitrification reaction.
2. Requirement of carbon for denitrification reaction.
3. Needs a lot of space for constructing separate chambers and involves intensive pumping operations.
Anammox the discovery:
In 1977, Ernest Broda stated, “Two kinds of litotrophs are missing in nature”.
He hypothesized that it must be possible to oxidize ammonium anaerobically with nitrite/nitrate as it’s thermodynamically feasible:
NH4+ + NO2- N2 + 2H2O ΔG = -358 kJ/mol NH4+
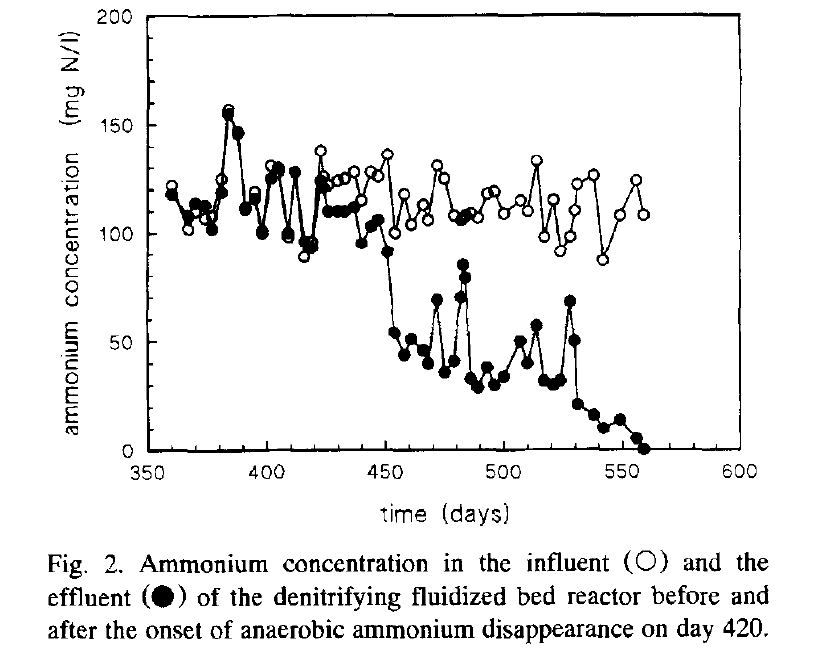
Anammox (anaerobic ammonium oxidation) remained only in theory until a team of Dutch engineers and researchers observed an unusual pattern in their denitrifying fluidized bed reactor. The figure below shows the ammonium concentration in the influent. All was normal till day 420. From day 420, they started observing a significant “disappearance” of ammonium in the denitrifying reactor. The observation leads to the discovery of anammox. Now we know that anammox accounts for over 50% of nitrogen turnover in marine environments, forming a critical component of the global biogeochemical cycle.
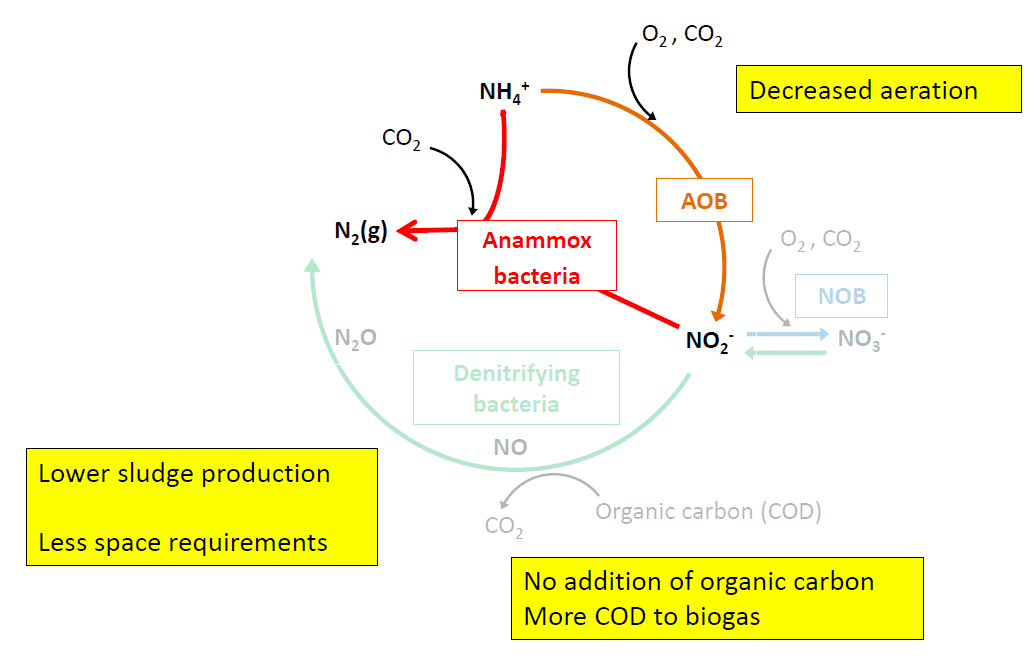
Advantages of the Anammox process:
1. Lower energy consumption – as the aeration required is reduced.
2. No external carbon is required.
3. Lower footprint. Complete nitrogen removal can be performed in a single bioreactor.
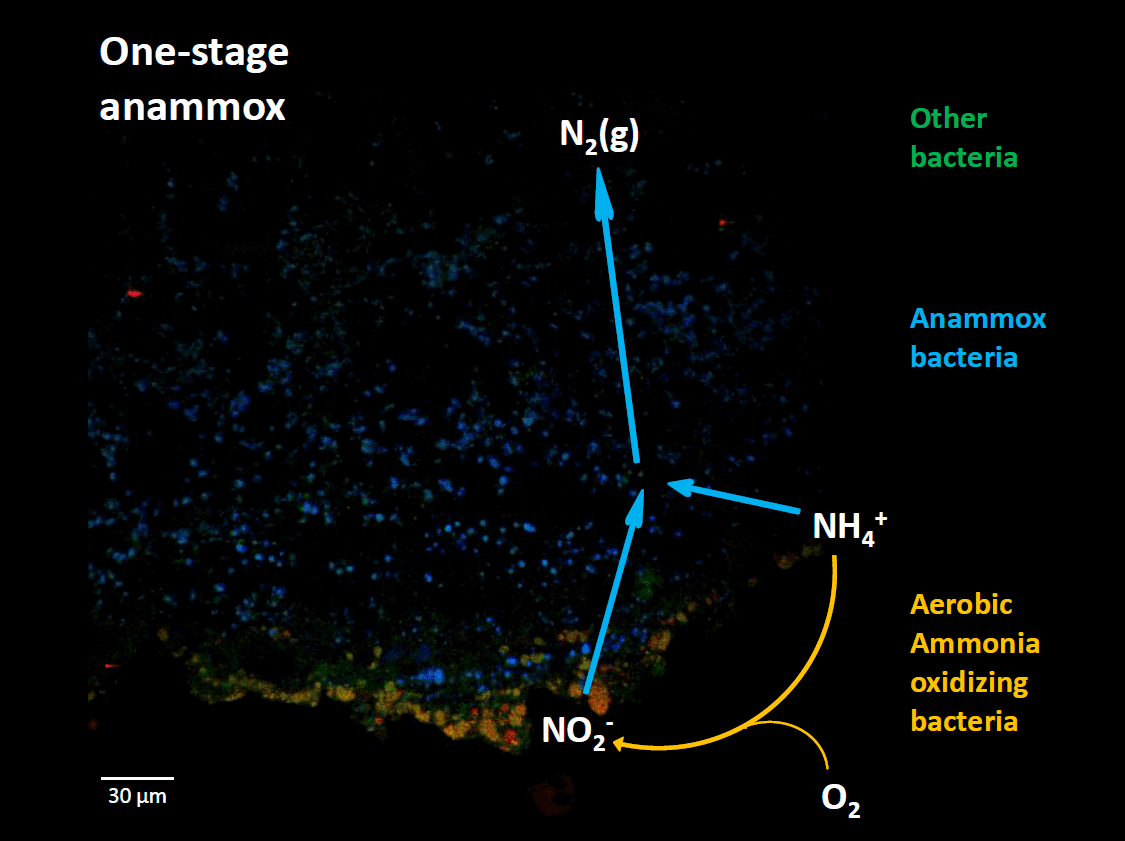
Credits: To my Guru, Prof. Frank Persson, Chalmers University of Technology, Sweden.
The following paragraphs are an excerpt from the breakthrough paper “Anaerobic ammonium oxidation discovered in a denitrifying fluidized bed reactor”.
Before Anammox - the problem:
Nitrogen removal from wastewater is critical for safeguarding sensitive water bodies. Nitrogen is often a rate-limiting compound for algae growth in surface water bodies. Nitrogen in wastewater when discharged into a water body, it makes it eutrophic. Eutrophication further leads to algae blooms.
Conventional biological nitrogen removal is a two-step process as discussed in FAQ-8. The first step is nitrification and the second step is the denitrification process. Nitrification in itself is often observed as a two-step process. The first step involves the conversion of ammonium into nitrite by ammonia-oxidizing bacteria/archaea. While the second step involves the conversion of nitrite to nitrate by nitrite-oxidizing bacteria. The first step of nitrification is a bottleneck, as the ammonium oxidizers are slow-growing autotrophs. And they compete with fast-growing heterotrophs for oxygen (electron acceptor). The denitrification process involves the conversion of produced nitrate to nitrogen gas. The bottleneck with the denitrification process is that it requires a carbon source (electron donor) and must be supplied. Nitrification and denitrification require different redox conditions i.e. aerobic and anoxic respectively. In wastewater treatment plants, separate chambers are required for each of these processes and the wastewater is cyclically pumped through these chambers.
Summary of the problems with conventional nitrification-denitrification:
1. High requirement of oxygen for nitrification reaction.
2. Requirement of carbon for denitrification reaction.
3. Needs a lot of space for constructing separate chambers and involves intensive pumping operations.
Anammox the discovery:
In 1977, Ernest Broda stated, “Two kinds of litotrophs are missing in nature”.
He hypothesized that it must be possible to oxidize ammonium anaerobically with nitrite/nitrate as it’s thermodynamically feasible:
NH4+ + NO2- N2 + 2H2O ΔG = -358 kJ/mol NH4+
Anammox (anaerobic ammonium oxidation) remained only in theory until a team of Dutch engineers and researchers observed an unusual pattern in their denitrifying fluidized bed reactor. The figure below shows the ammonium concentration in the influent. All was normal till day 420. From day 420, they started observing a significant “disappearance” of ammonium in the denitrifying reactor. The observation leads to the discovery of anammox. Now we know that anammox accounts for over 50% of nitrogen turnover in marine environments, forming a critical component of the global biogeochemical cycle.
Advantages of the Anammox process:
1. Lower energy consumption – as the aeration required is reduced.
2. No external carbon is required.
3. Lower footprint. Complete nitrogen removal can be performed in a single bioreactor.
Credits: To my Guru, Prof. Frank Persson, Chalmers University of Technology, Sweden.